The neuromatrix theory of pain is currently accepted among most pain researchers and clinicians as an explanation to how pain works, what it is, and what it means to most people with different types of pain.
It includes psychological factors that influence how we feel pain, such as emotion and cognition. It also serves as a guideline for clinicians to decide which treatments and care are best for a particularly patient.
Developed by the late Dr. Ronald Melzack (1929-2019) in the 1980s, the neuromatrix theory of pain expanded his previous gate control theory, which revolutionized pain management and the understanding of pain worldwide.
Despite the acceptance of the neuromatrix theory of pain, much of the education for massage and other manual therapies in many countries still teaches the biomechanical and biomedical models of pain.
These teachings often strongly associate pain with “poor” posture, “dysfunctional” movements, the degree of tissue damage, leg length discrepancy, “tight” muscles or fascia, and many structural factors.
The neuromatrix theory of pain has strong roots in neuroscience and psychology, provides a more complete and “less wrong” narrative behind what is pain.
By understanding the neuromatrix theory, therapists should be able to weave an updated narrative behind their hands-on work, recognize other factors other than “pushing skin” or stretching muscles, and question the traditional models and narratives that were taught in their early education.
What is the neuromatrix theory of pain?

The pain matrix is a representation of our pain experience process, a blend of biological, cognitive, and emotive factors that produces a “neurosignature,” similar to a fingerprint. (Image by Nick Ng, via MidJourney.)
While the gate control theory of pain is the first pain theory that shifts the focus away from the peripheral nerves and toward the spinal cord and the brain, it’s still heavily based on biology with the sprinkle of psychology. The neuromatrix theory attempts to fill in gaps that the gate control theory couldn’t fulfill.
Basically, Melzack describes the neuromatrix as a network of neurons in the brain that loops between the cerebral cortex (the large outer mass of the brain) and the thalamus and the limbic system, sort of like a subway system. This “loop” is different for everyone because we all have unique experiences, environments, social upbringings, and genetics, which create our personal neurosignature.
The neurosignature is the pattern of neural impulses that the neuromatrix produces. What we feel as pain—acute or chronic— is made up of biological (e.g. hormones, immune system), emotional, and cognitive factors.
Thus, how we sense pain and respond to it depend on such complex interaction: our biology, how we feel, what we think and believe, and our environment (e.g. home, work, family, peers, government).
See link for Melzack’s illustration of the neuromatrix.
Neuromatrix theory of pain example
Let’s say that you are hiking in a forest somewhere in Northern California, and you spot a mountain lion about 50 meters in front of you. Unfortunately, the lion sprints toward you with her fangs bared. You instinctively turn and run as fast you can. A few seconds later, you trip and tumble down a hill.
When you crash at the bottom of the hill, you get up and continue to run along a dirt path for another two minutes before you stop, look behind you, and catch your breath. You don’t see the mountain lion and you hear nothing other than birds chirping in the trees.
When you finally find your way back to your campsite and tell your camping friends about what happened, you start to feel a throbbing pain in your right ankle. You take your boot and sock off and see that your right ankle is bruised and swollen. You may have twisted it while you were running or were falling down the hill.
Either way, the pain in your ankle increases while your friends give you a first-aid treatment.
If we were to apply the traditional narrative of injury or tissue damage equating to the degree of pain, then it wouldn’t really explain why you were able to keep running despite having an ankle injury.
The gate control theory would explain that while you were under the stress of being killed and eaten, there’s a “gate” in the spinal cord that blocked sensory information from the injured ankle to the brain, which allowed you to “ignore” the pain and run from danger.
When you reached the campsite and saw your friends, they were a sign of safety. There was no need to gate the injury any longer and the ankle pain told you that your ankle needs to be attended.
The neuromatrix theory of pain adds that the safety of the environment (campsite), first-aid support and protection from friends, and not seeing the mountain lion anywhere all influence the neuromatrix in your brain, which affects how your neurons communicate that gives you the neurosignature. This neurosignature would change throughout your recovery.
Whether or not your ankle pain becomes persistent months later—even if it’s completely healed and you’re able to run and frolic again—it would depend on how well you healed, the type of support you had, how you coped with the mountain lion encounter, and if there are other stressors that affect your recovery.
Stories like this are also quite common among injured soldiers and athletes who don’t sense the injury or feel pain until they’re out of danger.
Biology behind neuromatrix theory of pain
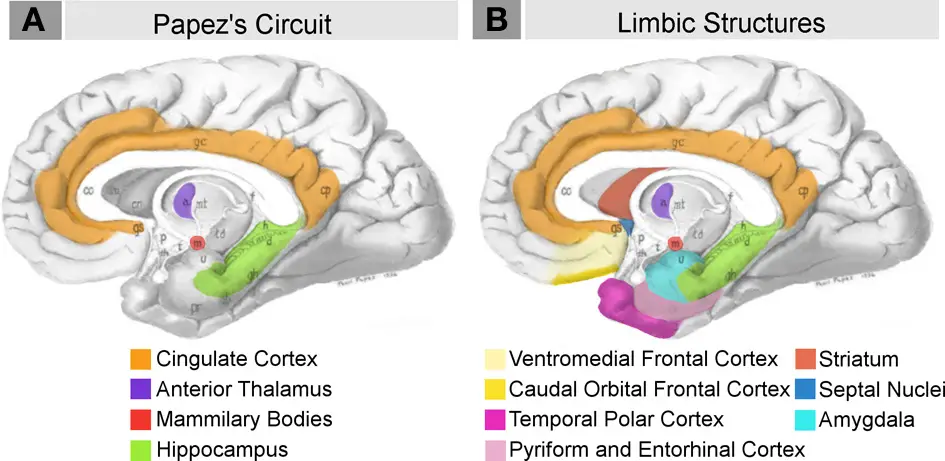
Highlights of the midbrain that contributes to the neuromatrix. (Photo: “Neural systems proposed to process emotion.png” by Barger N, Hanson KL, Teffer K, Schenker-Ahmed NM and Semendeferi K is licensed under CC BY 3.0.)
Currently, the brain regions that are associated with different types of pain are the primary and secondary somatosensory cortex (S1, S2), anterior cingulate cortex (ACC), insular cortex, thalamus, and prefrontal cortex (PFC).
These all make up the “pain matrix.” These findings, however, are still relatively new—less than 100 years old—compared to more than two centuries of examining pain mostly through the peripheral nervous system lens.
The biology of the neuromatrix theory stems not only from the gate control theory of pain but also from previous pain research in the 1800s to the 1950s. Many early beliefs about pain in various cultures include “energy forces” based on vitalism or divine powers from a deity.
French philosopher René Descartes proposed a non-divine or spiritual explanation for why we feel pain in the 17th century with remnants of vitalistic features. A classic depiction would be the illustration of a young man moving his foot away from a fire where “pain signals” from the foot travel through the leg and body and into the brain, which then drives the movement.
Physicians and physiologists in Europe and North America from the late 18th- to the mid-20th centuries constructed and refined Descartes’ idea and developed several pain theories that tell a little more about what pain is and how it works.
These include the specificity theory of pain, summation theory, intensity and pattern theories, sensory interaction theory, and eventually, the gate control theory.
Other than the gate control theory, these pain theories focus primarily on the peripheral nerves, but as each pain theory is refined, the focus shifts closer to the central nervous system and the brain.
Sensory
The neuromatrix theory of pain doesn’t discount the role of peripheral nerves and the skin. It tries to level the playing field by incorporating psychology into biological explanations. Still, sensory input is an important factor in the neuromatrix, which includes sensory input from the peripheral nerves, visceral organs, and our five primary senses.
Nearly a decade before the gate-control theory of pain was published, there were a few studies that are worth mentioning that boosted ideas for Melzack and Wall.
Dutch neurosurgeon Willem Noordenbos (1910-1990) proposed the sensory interaction theory of pain, which assumes there are two systems of how pain is sensed. This is from his research among patients with herpes zoster who felt extreme pain when they were gently touched.
- Slow unmyelinated and small myelinated afferent fibers system that sends nociceptive messages to the spinal cord where the cells in the dorsal horn transmit them to the brain;
- Fast myelinated system that conducts other sensory stimuli from the peripheral nerves and skin to the dorsal horn. This prevents the small fibers from transmitting pain messages to the spinal cord.
Noordenbos hypothesized that the diseased, large, myelinated afferent fibers prevented them from doing their job of inhibiting the slow and small afferent fibers. Therefore, any damage to the function of the fast myelinated system can lose our ability to control—or “gate” pain.
Also, several research by Frank, Fuortes, J.C. Eccles, and Magni in the late 1950s and early 1960s found that large afferent fibers inhibited long-term blocking of the transmissions of presynaptic neurons (neurons that deliver the message) to motor neurons. These research and Noordenbo’s work set the stage for Melzack and his colleague, Dr. Patrick D. Wall (1925-2001) to further investigate and develop the gate control theory, published in 1965.
Melzack wrote in 1999 that Noordenbos’s pain theory “stops at the thalamus,” as if that’s all there is to pain sensation. He continued, “My idea was to put a cortex on Bill’s thalamus, show the dorsal column projection as a rapid, precise feedforward system to activate psychological processes, with a feedback to the circle to modulate the input.”
This is where the gate control theory of pain began to bridge biology with psychology, which eventually led to the neuromatrix theory of pain.
Enter the pain matrix
As Melzack described it, the neuromatrix is the network of neurons and synapses that loops through different parts of the brain. The repeated pattern of neural impulses forms the neurosignature, which are like fingerprints where none are exactly the same.
Therefore, how we experience pain and what triggers pain can be uniquely different as well. This may explain why certain types of chronic pain, like back pain, hip pain, and knee pain, can be difficult to treat.
Melzack said that this neurosignature is “projected” to the “sentient neural hub”, which is where a “stream of nerve impulses is converted into a continually changing stream of awareness.”
The neurosignature pattern also drives movements through the same networks that allow the spinal cord to control fine motor patterns or complex movements, allowing us to escape or deal with a threat, seek treatment for pain, or another action that addresses the pain.
Psychology behind neuromatrix theory of pain
In addition to the sensory component, Melzack included two psychological factors that are part of the neuromatrix: cognition and emotion.
Image by Nick Ng, via MidJourney.
Cognition
The cognition component of the neuromatrix is divided into two categories: tonic and phasic. In physiology, tonic usually refers to stimuli that are slow to adapt and often continue to produce diminishing neural impulses even when the stimuli stop. It is constant like a steady stream trickling down a rocky cliff.
In terms of cognition, this refers to behaviors that are more difficult to change and “hang out” in the back of our mind. These include our past experiences of pain, our knowledge and attitude toward pain, and how our culture and upbringing shapes our perception of pain.
Phasic behaviors are the opposite where they can rapidly adapt and change according to different stimuli and environments. Examples of these include depression, anxiety, expectations, and focus.
Emotion (affect)
The affective aspects of the pain matrix cannot be separated from the biological components because they are constantly affecting each other. When we feel sad, happy, scared, or angry, our brain and various glands in our body produce hormones that influence our thoughts and actions.
Back to the mountain lion example, when you are under stress of being killed and eaten, your adrenal cortex in your kidneys produces glucocorticoids, a hormone that quickly breaks down some muscles tissues into glucose for immediate energy usage to fuel your muscles as you take action during a “fight or flight” response. When you escape from the mountain lion and you are in a safe environment, your body stops producing glucocorticoids and attempts to restore homeostasis.
In chronic pain, Melzack proposed that it may be the result of a combined effect of elevated cortisol levels in the body, which is another stress hormone. He pointed out that the hippocampus in the brain—a region that is associated with forming memories and regulating our emotions and learning—tend to lose nerve fibers and size as people age.
Since the hippocampus is the gatekeeper of cortisol release, this loss reduces the “natural brake” and causes a chain reaction that leads to chronic pain and the development of secondary conditions, like fibromyalgia and arthritis. Social and hormonal factors can also worsen the existing condition: loneliness and isolation, genetic predispositions, daily stress, and changing sex hormones.
“The nervous system might only be two percent of your body, but it runs 100 percent of ‘you’, so you will often know if things aren’t feeling quite right,” said Diane Jacobs, who is a retired physical therapist in Weyburn, Manitoba, and the author of “Dermoneruomodulating.”
She says that every input into the nervous—your experiences, what you learned, memories, how you think, how you were treated—are all embodied into your sense of self.
“The innovative thing about this framework is that Melzack didn’t line up pain as an inevitable result of nociceptive sensory input the way other models do,” Jacobs said. “He separated them and pointed out that nociception is input into a neuromatrix, and pain is an output from a neuromatrix. The good part about this is that there are so many levers to pull in here and you can start to examine your life and see where you might be able to exert control and change certain aspects of your life to help you deal with your pain.”
[An archive interview with Dr. Ronald Melzack from McGill University.]
Problems with the neuromatrix theory of pain
Since Melzack had published his paper on the neuromatrix theory of pain, there have been some questions about the nature of the “pain matrix” and its definition.
In 2007, two French physicians, Xaiver Moisset and Didier Bouhassira, reviewed several studies that examined how various human brains respond to nociceptive input. They found that not all brain regions associated with the neuromatrix “light up” when subjects feel pain.
In two of the studies, Moisset and Bouhassira highlighted that there was no change in blood flow in S1 and S2 of the brain, but there was an increase of such flow in the insula and ACC in one of the studies. This may indicate that chronic pain is related to changes in brain regions “associated with the affective/emotional dimension of pain.”
But there were conflicting results from other studies cited, such as those that examined allodynia, which is having pain from stimuli that normally don’t elicit pain, such as being stroked with a soft paintbrush. In most of these cases, allodynia is associated with injury to the central nervous system (spinal cord or brain) or peripheral nerves. Thus, this condition cannot be explained by the pain matrix alone.
After reviewing seven studies that focused on this type of allodynia, Moisset and Bouhassira stated that “it is unclear whether the brush-evoked allodynia associated with different types of lesions results from similar mechanisms in all cases. The differences in responses among the subject provided clues on how the somatosensory cortices process the incoming stimuli from the peripheral nerves.”
The ACC, which is part of the limbic system that deals with impulse control, reward anticipation, morality, and various emotions, has reduced activation among patients with Wallenberg syndrome and various types of brain trauma injury.
This is a rare condition where there’s a stroke in the lateral medulla of the brain stem. Moisset and Bouhassira found that the patients in the Wallenberg studies had various brain activation, but given the different study methods, small sample sizes, and bias in the literature they reviewed, they cautioned that any conclusions should “remain tentative.”
Currently, the definition of “neuromatrix” also deviated from what Melzack had proposed. When researchers failed to find a specific region of the brain that processes pain, the neuromatrix term was developed to offer an alternative explanation. Iannetti and Mouraux wrote that the neuromatrix isn’t just about pain but a reference to “many possible perceptual outputs.”
It constitutes both nociceptive and non-nociceptive inputs that use different brain regions to create the neurosignature and “body-self” that Melzack proposed, who wrote in 2005, “…the neuromatrix, distributed throughout many areas of the brain, comprises a widespread network of neurons that generates patterns, processes information that flows through it and ultimately produces the pattern that is felt as a whole body possessing a sense of self.”
Iannetti and Mouraux argued that pain is only one output among many that is the product of the “pain matrix.” Also, increased intensity of the nociceptive stimuli doesn’t necessarily correlate to increased perceived pain.
For example, in a 2008 British study by Iannetti et al., seven subjects (five men, two women) were zapped on the back of their hand with a beam of laser three times for one second at increasing intensity intervals. The pain sensation “does not affect the intensity of the elicited pain sensation.”
Because imaging studies do not find that responses to painful stimuli in specific areas of the pain matrix, Iannetti and Mouraux suggest that the neuromatrix, as originally proposed by Melzack, is “activity of a multimodal neural network.”
Pain is one of the reactions of this network that is on a constant lookout for relevant information from the environment, whether it is danger, sustenance, companion, etc., to ensure the safety and survival of an organism.
“In the early years, [Melzack] had trigger points in there as one of many sensory inputs, which are these unicorn things that no one but true believers can find through palpation and that they then go on to stab with needles,” Jacobs said. “Melzack cleaned up his diagram as years went by, and took away a lot of words, including that one, leaving a much cleaner looking framework that people can fill in with their own life and trauma history and body history.”
Beyond the neuromatrix theory and the biopsychosocial model of pain
As with any scientific theory, it would eventually be replaced by a new theory, just like how Albert Einstein’s theory of relativity replaced Issac Newton’s theory of universal gravity when explaining how planets revolve around the Sun.
Likewise, the neuromatrix theory of pain provided a more accurate (or less wrong) explanation about pain than the gate control theory, and in turn, the gate control theory had replaced previous theories of pain (e.g. summation theory, specificity theory).
While the gate control theory of pain was just starting to hit medical textbooks in the mid-1970s, psychiatrist George L. Engel (1913-1999), who taught at the University of Rochester Medical Center, introduced the biopsychosocial (BPS) model of healthcare. This model arose from the neglect of consideration of the patient’s psychology and social needs at the contemporary biomedical model.
Some may think that the BPS model of healthcare would match most of the tenets of neuromatrix theory since the latter also includes psychosocial factors. Recently in 2019, another idea (not theory yet) may further expand the BPS model of pain and perhaps even the neuromatrix theory: the enactive approach to pain.
Dr. Peter Stilwell of McGill University in Montreal, Ontario, and Dr. Katherine Harman from Dalhousie University in Halifax, Nova Scotia, described the enactive approach as “the bigger picture” of pain where the BPS model of pain continues to “perpetuate dualistic and reductionist beliefs.” It’s drawn from the teachings of philosopher Shaun Gallagher, who developed the “4E” as a novel way to understand the human mind.
Briefly, the 4E stands for:
- Embodied: recognizing that the body is an important part of consciousness and the human experience.
- Embedded: how the environment affects a person’s perception of safety or danger, which predicts actions.
- Enacted: based on the idea that living organisms are “self-creating, self-maintaining, precarious” and “adaptive,” this approach is an attempt to find meaning to a situation or environment (e.g. back pain).
- Extended: based on the idea that the environment is an extension of the mind. This is where organisms engage with their environment, from using a pen, car, or smartphone, to government institutions and civil right movements.
Stilwell and Harman suggested a fifth E: emotive. This is tying the emotion and cognitive components of pain, which is also mentioned by Melzack in the neuromatrix theory, rather than address both as separate entities. Interpreting meaning to a stimulus or situation is not only a cognitive process but also how it is felt.
Whether or not the neuromatrix theory of pain can be critiqued in such a way is debatable, and the enactive approach to pain may be the next step in formulating a new theory.
“…the BPS model of pain is not perfect. Any model our minds can conceive of is potentially flawed and biased towards what we currently know. But it is the best explanatory model of pain that we have thought of with our current and potentially flawed knowledge base.” ~ Lars Avemarie, PT (Source)
[Read: Realism and Theory Change in Science]
“I discussed how it opened up the field away from the standard orthodox biomedical models of pain being an endpoint of nociception in a straight line,” Jacobs said, in reference to the neuromatrix theory. “Reality is a lot more convoluted. I found the neuromatrix framework simple, elegant, without leaving out anything.
“When I worked with patients, I had the model printed off and would sometimes pull out a sheet and sit down and go over it with the patient and we would write down specific things in the domain areas that might be contributing factors. I would give them a copy to take home in case they thought of other things to add.”
Although Melzack’s description of the neurosignature is questioned by some scientists and clinicians, the gate control theory and the neuromatrix theory of pain impacted how the medical community and manual therapy education think and frame about pain.
“Never again, after 1965, could anyone try to explain pain exclusively in terms of peripheral factors,” Melzack wrote. “The theory forced the medical and biological sciences to accept the brain as an active system that filters, selects and modulates inputs.”
A native of San Diego for nearly 40 years, Nick Ng is an editor of Massage & Fitness Magazine, an online publication for manual therapists and the public who want to explore the science behind touch, pain, and exercise, and how to apply that in their hands-on practice or daily lives.
An alumni from San Diego State University with a B.A. in Graphic Communications, Nick also completed his massage therapy training at International Professional School of Bodywork in San Diego in 2014.
When he is not writing or reading, you would likely find him weightlifting at the gym, salsa dancing, or exploring new areas to walk and eat around Southern California.