If you’re a massage therapist, you probably have noticed that different patients or clients have responded differently to your treatment. Some prefer lighter or deeper pressure, and some prefer sustained pressure or stretching while some do not.
So why is that? Is there something going on in the tissues and nerves? Hormones? The brain?
Touch receptors in the skin and its neurobiology don’t fully explain why different people can react differently to the same kind of touch, from pleasure to disgust. As some research has shown, context, culture, and various social and environmental factors also affect how we perceive touch.
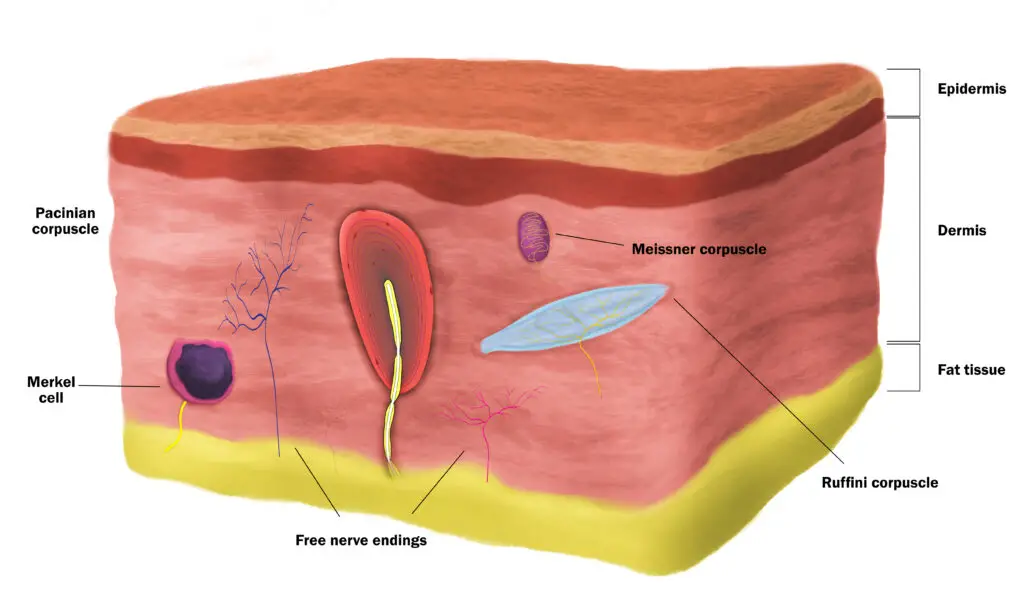
Different types of touch receptors in the skin detect different stimuli from your environment. (Illustration by Nick Ng.)
Touch receptors in the skin
Living organisms constantly sense their surroundings—particularly touch for mammals—for survival and development. Within your skin are three main kinds of sensory receptors: Mechanoreceptors, thermoreceptors, and nociceptors. Each type works together to gather information about our environment with the immune and endocrine systems.
Senses that detect stretch, temperature, pressure, and vibration constantly inform us if we’re safe or not. If you feel a prick on your shin, you might rub or scratch the spot and examine if something had bitten you.
Different areas of your skin contain different numbers of certain types of touch receptors. High-sensitive neurons that can quickly distinguish different sensory input, such as feeling the difference between silk and polyester fabrics, are clustered more at your fingertips than on your back or forehead.
Mechanoreceptors
When you’re poked, pulled, pushed, or brushed, mechanoreceptors react to its physical changes and the surrounding tissues. How you feel and respond depends on the amount and speed of the pressure and the context of touch.
Lanceolate endings
These receptors, which wrap around hair roots, respond to light touch and movement on the skin, ideal for detecting something crawling or moving on your skin. Some evidence shows that some people tend to prefer a “moderate” rate of brushing compared to slow or quick strokes, particularly at the thighs, shin, forehead, and forearm.
Lamellar corpuscles (Pacinian corpuscles)
Lamellar corpuscles detect changes in broad areas of your skin and higher frequency vibrations than other mechanoreceptors, even if there’s no contact with the skin.
Tactile corpuscles (Meissner’s corpuscles)
These receptors are also sensitive to light touch, but they’re more sensitive to changes in shape and texture and are located closer to the skin surface than lamellar corpuscles. This helps you differentiate tiny differences in textures, such as Braille. Tactile receptors also are good in detecting low-frequency vibrations.
Merkel cells
While these receptors are similar in function as tactile corpuscles, not much is known about what Merkel cells do. However, they are more uncommon than other receptors, localized mostly in your fingertips and can be cancerous.
Bulbous corpuscles (Ruffini corpuscles)
These receptors are sensitive to changes of skin stretching and changes in the position of the hands and fingers. Bulbous corpuscles also detect grip strength and whether something will slip from your hands.
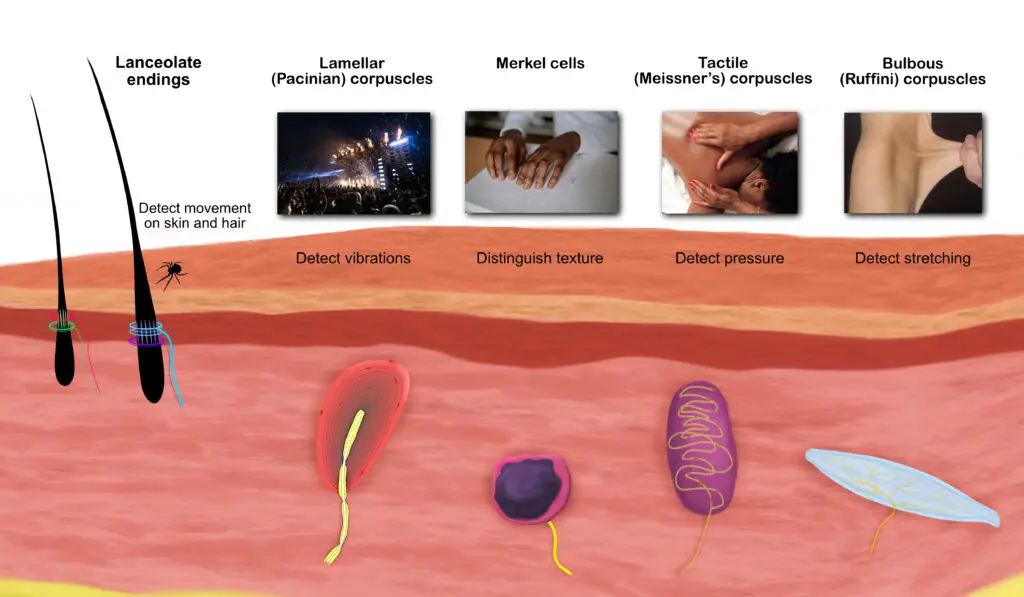
Touch receptors work together to gather information about our environment. These include the lanceolate endings, lamellar corpuscles, tactile corpuscles, bulbous corpuscles, and Merkel cells. (Illustration by Nick Ng)
Thermoreceptors
Located in your skeletal muscles, tongue, some internal organs (e.g. liver, bladder), hypothalamus of your forebrain, and skin, thermoreceptors are free nerve endings that detect changes in temperature within your body and environment. When temperature reaches to a point where you begin to feel discomfort, it may elicit pain.
Like most mechanoreceptors that adapt to pressure changes, thermoreceptors can adapt to temperature changes, but there’s a range that these skin receptors can adapt to.
Heat receptors adapt within the range of 29°C (84° F) to 45°C (113°F).
Cold receptors fall within 5°C (41°F) to 40°C (104°F).
For most people, thermoreceptors cannot adapt to temperatures outside of these ranges.
Nociceptors
Nociceptors are a mixed bunch of sensory neurons that detect and respond to internal and external stimuli, such as temperature and touch. Being specialized sensors, some of them will respond to mechanical changes, such as a pinch or poke, but not temperature changes. This is because different types of nociceptors are innervated by different nerve fibers.
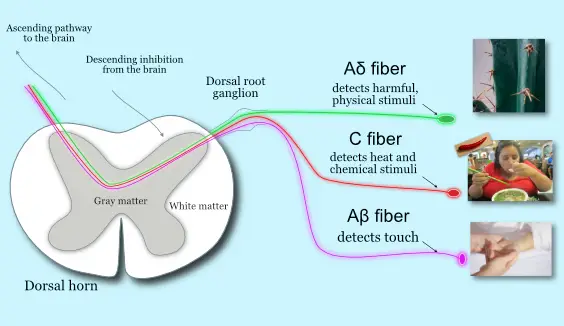
Different nociceptors detect different types of physical stimuli, and the brain and spinal cord “decide” if these input are dangerous or not. (Illustration by Nick Ng).
A fibers
Aβ receptors have thick bundles of myelin that respond best to light touch, like a brush from a feather or a slight skin stretch. They can also inhibit nociceptive input from Aδ and C-fibers.
Aδ fibers are unmyelinated, high-pressure threshold nociceptors that relay information from the peripheral nerves to the dorsal root ganglion (DRG) in the spinal cord, which is often associated with processing acute, subacute, and chronic pain. They also function as proprioceptors, sensing where joints and other body parts are located within your personal space.
Aδ fibers also respond to cold temperatures and acute pain and are associated with the classic withdrawal reflex with the DRG.
More rabbit holes: gate control theory of pain, neuromatrix theory of pain
C fibers
C-tactile fibers, like Aδ receptors, are also unmyelinated, but they’re the secondary responders to pain because they require deeper pressure to elicit a response than Aδ.
Some of the major sensory nerves that allow us to respond to the type of touch. Not all skin surfaces contain the same amount of receptors. C-tactile fibers, for example, are more common along arms and legs than the abdominal region. Evolutionarily speaking, this makes sense because we humans rely mostly on our limbs to gather tactile information from our environment.
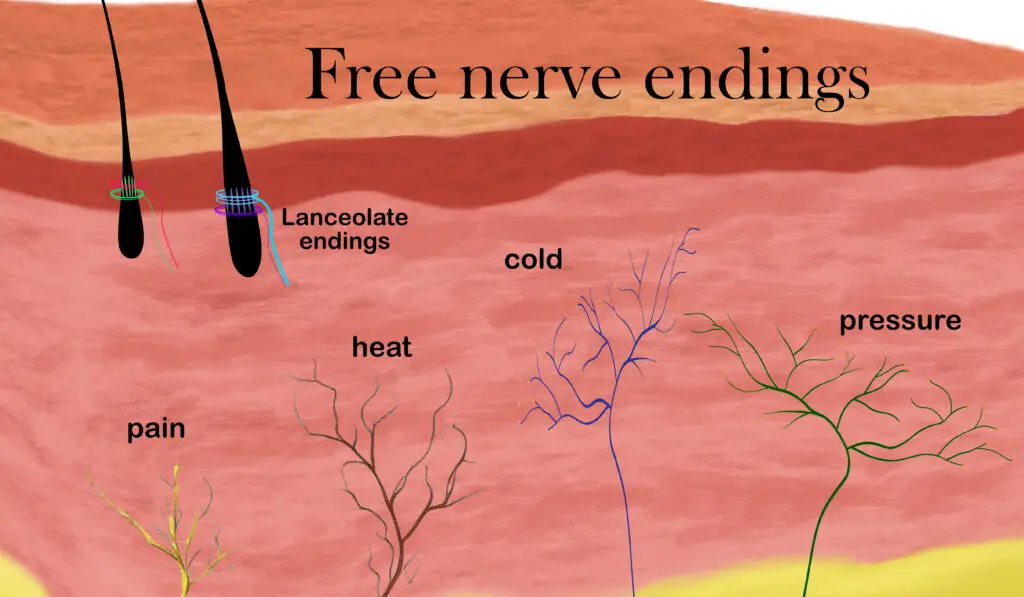
Several types of free nerve endings detect temperature, pressure, itch, and danger. (Illustration by Nick Ng.)
How the central nervous system and skin processes touch
Despite having different roles, all receptors operate on a basic blueprint. When someone touches you, sensory receptors transmit electrical signals to the DRG. If the touch is painful or unpleasant, nociceptors in the Rexed laminae I and II—which are two of the ten layers of the gray matter that process pain—relay these signals to other Rexed layers (IV and VII). This starts a process (ascending modulation) to the thalamus of the brain.
During this process, opioid receptors are activated in the presynaptic ends of nociceptors in Rexed layers IV and VII, which stops the activation and release of substance P, a neurotransmitter that modulates pain.
Then various parts of the brain process the information, including the thalamus, periaqueductal gray (PAG) in the upper brain stem, insula, prefrontal cortices, raphe magnus, locus coeruleus, and nucleus reticularis gigantocellularis contribute to pain inhibition via descending modulation.
In this process, many neurotransmitters are produced to suppress pain or simply increase the sense of pleasure if the touch stimulus is considered non-threatening. These neurotransmitters include serotonin, endogenous opioids, vasopressin, and oxytocin, which act as “painkillers.” All these events happen in milliseconds.
Keratinocytes and pain modulation
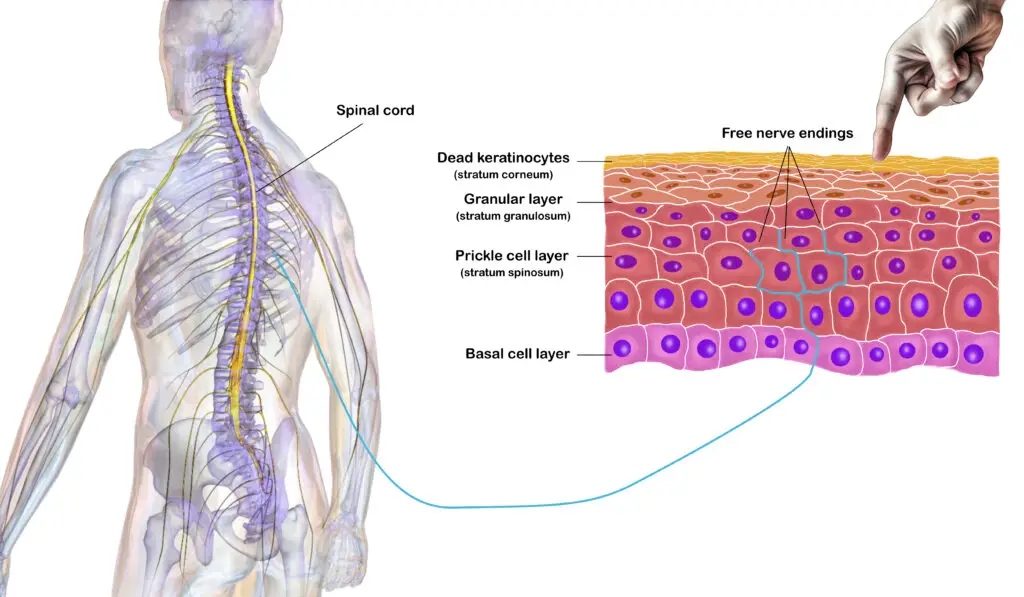
Keratinocytes play a role in sensing touch and pain, modulated by the spinal cord and brain. (Illustration by Nick Ng; spinal cord and human body by Blausen.)
Nerves aren’t the only ones that play a role in sensing touch and pain. They need a “messenger” to detect mechanical and other stimuli. Since your skin is made up of keratinocytes (cells that produce keratin, a protein that makes up our skin and hair), these skin cells are the first to make contact with our environment.
Researchers Moehring, Cowie, et al. from the Medical College of Wisconsin in Milwaukee found that when certain keratinocytes in mice are “turned off” under exposure to a low-voltage LED light at 30-second intervals, this lowers their response to touch stimuli.
Their research further validated previous research that pressure on the skin makes the keratinocytes release ATP (adenosine triphosphate), which binds to nerve cell receptors called P2X4. This is the first step needed for nerves to sense mechanical stimuli before the information is transmitted to the spinal cord and brain. However, when the researchers damaged their nerve receptors or prevented ATP attachment to the nerve cell, the mice did not respond much to touch—painful and non-painful types.
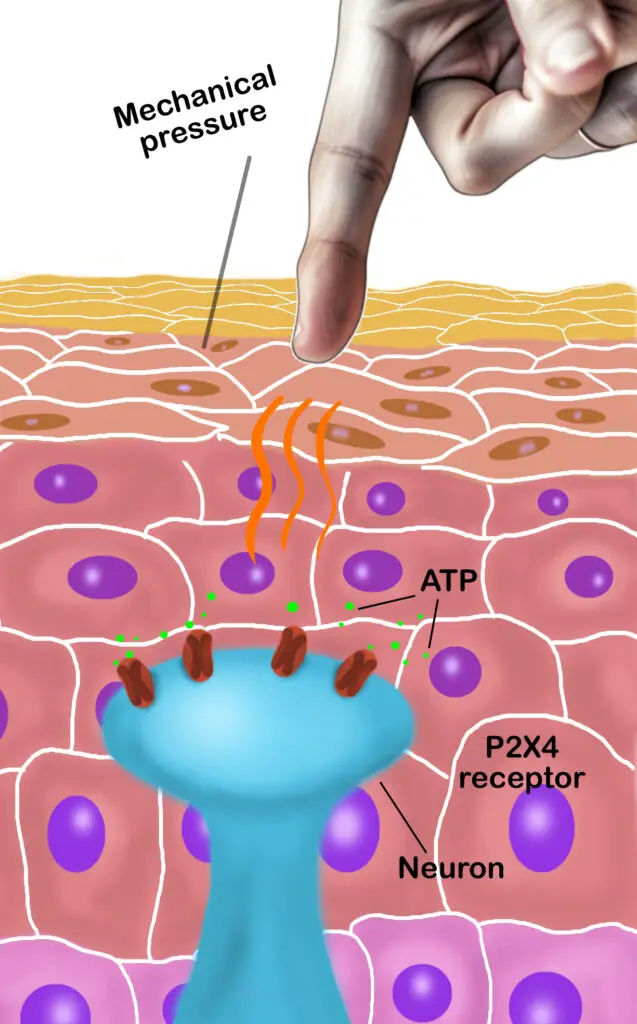
Neurons that detect touch has receptors called P2X4 that releases ATP, which goes through a process that sends messages to the spinal column and brain to determine the nature of the touch stimulus. (Illustration by Nick Ng.)
Although keratinocytes also release other chemical messengers that can activate nerve receptors, they found that ATP is the major contributor to starting the sensory “ignition” on non-injured skin. In injured or diseased skin, however, they said that there may be more factors other than ATP that increases nociception.
If this is true, this expands our understanding of the role keratinocytes play in how we experience pain and touch. They may provide sensory receptors with “numerous opportunities for initiation and amplification of signaling mechanisms that underlie pain, itch or dysesthesia in the setting of disease,” the researchers wrote.
Given what’s known about how we sense pain and touch, would descending modulation be sufficient to explain why some people tend to feel less pain after getting a massage or having some manual therapy?
“I think it is important to bear in mind that different mechanisms compete, complement, and interact. It is hard to say for certain that a single mechanism or set of mechanisms dominate the clinical response,” Andrew Vigotsky explained, who is a biomedical engineering Ph.D. candidate at Northwestern University.

“I believe it is certainly feasible that descending modulatory circuits play a role in the analgesic response that some individuals experience. I think the better question is, to what extent, in what individuals, and under what circumstances?” ~ Andrew Vigotsky (Photo courtesy of Andrew Vigotsky.)
“For instance, one may experience analgesia as a result of mechanism A which competes with that of mechanism B. After blocking mechanism A (e.g., using a competitive receptor antagonist), mechanism B may dominate. Clinically, the difference may be undetectable, and as a result, connecting mechanisms to clinical outcomes would be difficult to study. That said, I believe it is certainly feasible that descending modulatory circuits play a role in the analgesic response that some individuals experience. I think the better question is, to what extent, in what individuals, and under what circumstances?”
How context affects sensory receptors and touch
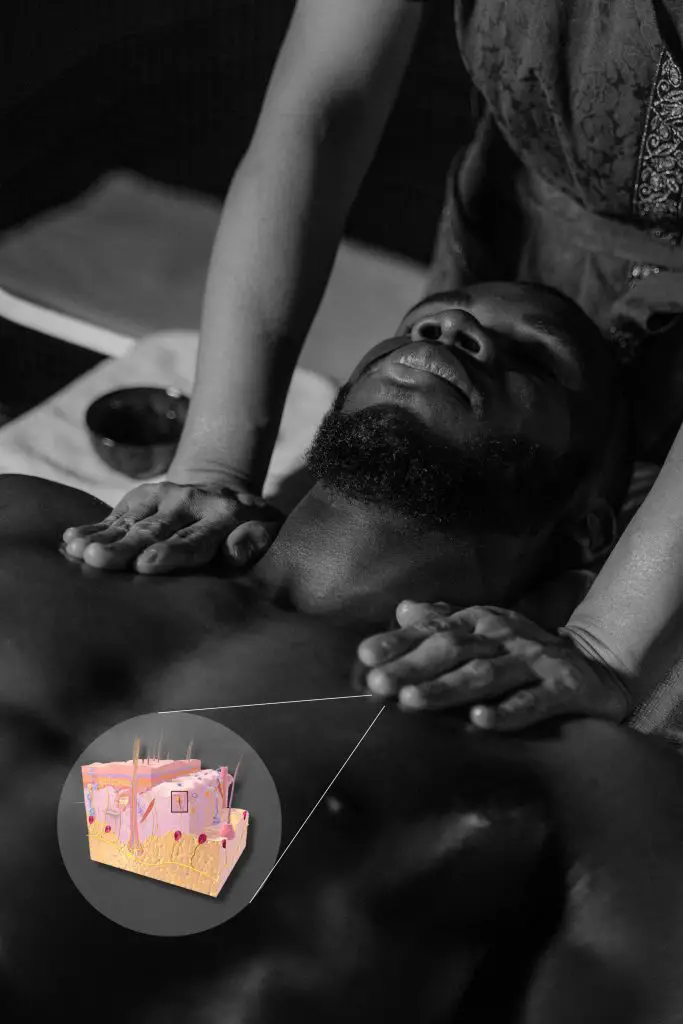
Context is important when it comes to how we process touch. (Photo by Tima Mroshnichenko. Skin illustration by Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436.)
Keratinocytes and touch receptors aren’t the only things that influence pain and touch. In the past decade, some researchers have found that you can respond differently to the same quality of touch based on context.
For example, in a 2012 study that took place at the California Institute of Technology, researchers wanted to see how much the primary somatosensory cortex (S1) in the brain processes the pleasantness of social touch.
They recruited 18 heterosexual men and had them put on a virtual reality-like goggles that show who will be doing the massage. The video showed either a slim woman in a black evening gown with a welcoming demeanor or a man in a black tank top and blue jeans who had a cold, standoff-ish behavior. The men could not see the actual person who is in the room with them since they were faced down. At some point in the video, the actress or actor moved to the side of the screen and “caressed” the subjects’ legs for a few seconds, but they could not see their own leg in the video or the caressing action itself.
Meanwhile, they could feel the touch that synchronized with the action in the video where the experimenter in the room synced their movements with the video. Each subject underwent four trials where they see the person “caress” and feel the caress and see the person “caress” with no actual caress.
While none of the subjects actually saw who did the touching, the experimenter is the same woman who did all the caressing. Everyone gave the actor a negative rating and the actress a positive one.
The researchers found that the S1 responded the most to gender differences to touch compared to the anterior cingulate cortex (ACC), insula, and secondary somatosensory cortex (S2).
To the researchers’ delight, the S1 got even more excited when the subjects saw the actor or actress move their hands off-screen yet they did not receive any touch. It seems that the anticipation of touch can excite the person as well as receiving actual touch. This is probably because of dopamine, the hormone that’s associated with emotions of reward and anticipation of reward. It peaks not when the reward is received but when the subjects see the cue that leads to the reward.
A similar study was done in 2014 in Germany where 40 heterosexual men were to respond to a female or male experimenter’s touch. The researchers wanted to see how oxytocin—often dubbed as the “love hormone”— affects social touch response. First, half the group received three puffs of oxytocin spray up their nose, and the others received a placebo (table salt water solution). Instead of wearing goggles like the other study, they were put inside a MRI scanner with either a male or female experimenter in the room whom they had met earlier when they entered the room. Like the previous experiment, the same female experimenter touched all the subjects’ lower leg under certain cues.
However, the subjects couldn’t see who touched them and believed that they were being touched by the same experimenter they had met earlier. Although all rated the female touch to be more pleasant than being touched by a male, those who received the oxytocin had a higher increase in the pleasantness than the placebo group where both groups believed they were touched by a woman.
However, there was no significant increase of pleasantness in any group when they think they were touched by a man, not even those with an oxytocin boost. Surprisingly, men who had autistic traits did not have a higher increase in pleasantness when they think they were touched by a woman.
This raises some questions that go beyond the biology of touch: how much do culture, social norms, and language affect our interpretation of touch? And how do these affect our biology and psychology?
Expectations and context on sensory receptors
Cognitive neuroscientist Dan-Mikael Ellginsen from the University of Oslo in Norway led a review that highlighted three ways that we experience touch.
The first way is called the gate of attention, which refers to where the focus of your body is. It’s basically your brain’s way of selecting specific stimulus to pay attention while filtering out others.
For example, clients might not realize they have a “sore spot” until you touch that area. Another more mundane example would be if you’re sitting on your couch watching a movie or working on your laptop, you probably aren’t aware of your seat pressing against your bottom or even how your feet and legs are positioned unless you bring awareness to those body parts.
The second way is prediction, a way for you to anticipate what something feels like before you even touch it. One great example in massage therapy is how some clients expect a massage would feel based on their prior experience.
What worked in the past for that person may likely work in the present. If they find deep tissue massage alleviates their back pain, then it’s likely beneficial to them. Conversely, we may imply that lighter touch methods work for those who find pain relief from such work.
The third way is context, such as visual and auditory cues from who is touching you, how you feel, and where your environment is. Being touched during a massage or physiotherapy session by a qualified therapist is quite different than having a random customer touch you at a coffee shop. You probably would not expect or accept that!
Sometimes C-tactile fibers do a “hedonic flip” where the same soothing touch can feel very threatening, or at best, uncomfortable. A common example is how sensitive the skin gets around an injured or inflamed area. Such clues support the idea that the change of C-tactile fiber function motivates you to protect the injured part.
“Given the strong contextual influences on touch pleasantness, it is unknown whether there are qualities of certain touch stimuli that inherently carry a positive hedonic value (i.e., are pleasant or give rise to positive affect), or whether the hedonic value of touch is always dependent on other contextual or internal factors,” Ellingsen et al. wrote. This may explain why the male subjects in the two studies mentioned earlier respond differently between being touched by a woman or man.
Can pain expectations from touch be changed?
Like most human behaviors, how we change our expectations of pain may influence the amount of chronic pain we experience. One major study unraveled a little bit of the mystery behind what people with chronic pain expect in the near-future, which was conducted from Arizona State University, led by Dr. Chung Jung Mun, who is currently a post-doctoral researcher at John Hopkins Medicine.
The researchers split the study into two sub-studies: Study 1 included 231 people who had rheumatoid arthritis and were told to keep a written diary of their pain experience before their bedtime. The participants received a package of 30 diary reports with 30 addressed and stamped envelopes to mail back to the researchers the next morning. Every night they rated their pain that day and wrote down what they expected their pain to be like tomorrow.
Study 2 included 220 people with fibromyalgia. Unlike the Study 1, participants were asked to assess themselves four times a day for 21 days via an automated system that called their cell phone. They responded to the recorded questions by using their phone keypad. Basically, they were asked to assess their social experiences each day and the pain’s impact on their social enjoyment and relationships.

People who expected pain to worsen at night are more likely to experience pain. (Photo by Greta Hoffman.)
“As expected, across different chronic pain groups, when participants reported a level of pain expectancy higher than their usual level, they were more likely to experience greater pain intensity the next day, controlling for the effect of today’s pain intensity,” Mun et al. wrote. “In Study 2, we found that increases in afternoon pain-related activity interference and positive affect were associated with higher evening pain expectancy. Pain expectancy, in turn, was related to impairments in the next afternoon’s social enjoyment as mediated by higher pain intensity experienced the next morning.”
They suggested that such expectations can lead to higher pain sensitivity in the central nervous system as well as stimulating cognitive and affective regions in the brain that increase anxiety.
Psychologists Martien Schrooten and Steven Linton from Örebro University lauded that study in Pain, suggesting that their research could expand to other disciplines, including those in exercise and physical rehab.
For example, patients with low back pain may have heard from their previous physician or a personal trainer that bending forward is bad for the back and may increase their pain condition. In reality, such movement may actually have very little or no damaging effects on the back, yet their beliefs and expectations make them hesitant to move or even to try to move. Thus, pain sensitivity increases to protect the back from a perceived threat when there isn’t really one. Schrooten and Linton also warned that correcting patients’ belief—even if they’re wrong—may backfire.
And so, expectations could worsen or improve how we experience touch and pain—with our nervous, endocrine, and immune systems being the major players.
“I am not sure that I can say that something is a primary contributor to pain modulation and sensation changes, but I think there is biological feasibility here for some effect,” Vigotsky said. “Like we allude to in our review, many of these neurotransmitters can bind to receptors in different parts of the nervous system, so what exactly they are doing and where remains unclear. In addition, I would like to see full factorial study designs to understand the effects and interactions of different mechanisms; I do not believe linear models of mechanisms hold much weight in physiology.”

Physiotherapist Richard Fernandez brushes Amy Thompson’s left hand, which is covered from her view, and a rubber left hand that simulates to be her real hand at the 2019 San Diego Pain Summit. Feb. 20, 2019. (Photo by Nick Ng.)
Nick Ng is the editor of Massage & Fitness Magazine and the managing editor for My Neighborhood News Network.
An alumni from San Diego State University with a bachelor’s in graphic communications, Nick also completed his massage therapy training at International Professional School of Bodywork in San Diego in 2014. In 2021, he earned an associate degree in journalism at Palomar College.
When he gets a chance, he enjoys weightlifting at the gym, salsa dancing, and exploring new areas in the Puget Sound area in Washington state.