When massage therapists explain pain to their patients or clients, they tend to tell their narrative based on the musculoskeletal system—and sometimes— the nervous system. While it is obvious that an ankle sprain or a knife wound is from tissue damage, our immune system are on the fringes of our peripheral tissues to serve as the first line of defense against disease and injury — even if the threat is not real.
For decades, researchers have known that our nervous system—including the brain—is responsible for many of our pain experiences. However, even that powerful system that shapes how we feel when our favorite sports team wins or when we lose a loved one does not tell a complete story of how pain works — especially chronic pain that persists for many months or years.
In early 2017, a team of researchers from Harvard Medical School published a review that examined how immune cells affect pain sensitivity, nociceptor activity, and inflammation. Nociceptors are specialized sensory neurons that are spread throughout our peripheral tissues, such as joints, skin, lungs and respiratory tracts, urinary tracts, and gastrointestinal tract. These areas are more sensitized than other regions of the body because they have direct exposure to the environment, an opening for pathogens to invade. It makes sense why such epithelial tissues are bundled with nociceptors.
These nociceptors are like a home security system that detects actual danger or potential threat from our environment. When we get hurt or an infection, the immune system releases mediators that sensitize nociceptors, which sends a series of “messages” called action potentials to the nociceptors within its dorsal root ganglia (DRG) of one or more vertebrae. They are relayed to the spinal cord and brain to be processed as pain, be it acute or chronic.
Of course, our brain can interpret these messages in different ways, and sometimes they get ignored because our brain does not perceive the threat to be dangerous enough to elicit pain. But under chronic pain conditions, even when no tissue damage exists, there is often some inflammation involved, which reduces the pain threshold and increase pain sensitivity.
Like immune cells and muscle fibers, nociceptors come in different types that perform different duties, depending on which layer of epithelial tissue they reside. For example, C-fiber nociceptors are non-myelinated, slow-conducting neurons that are mostly capsaicin sensitive and often mediate thermal pain sensitivity. (Capsaicin is a what gives us that burning sensation in our mouth when we bite into a serrano pepper, which is why sometimes we don’t detect the “heat” immediately.)
By contrast, fast responding, myelinated δ and β fibers sense mechanical pressure, allowing us to respond quickly to whatever is pressing or touching us, such as a spider crawling up our leg or a loved one caressing our hands.
Injury can stimulate a rise in the number of immune cells in nociceptors’ cell bodies within the DRG, which is the site where neuroplasticity takes place — a process where the nervous system reorganizes synapses in response and adaptation to learning, experience, or injury. This can also increase the amount of chemicals produced by immune cells that can further sensitize us for pain. For example, neutrophils release a chemical called leukocyte elastase in the DRG to induce pain after a nerve injury in the lungs.
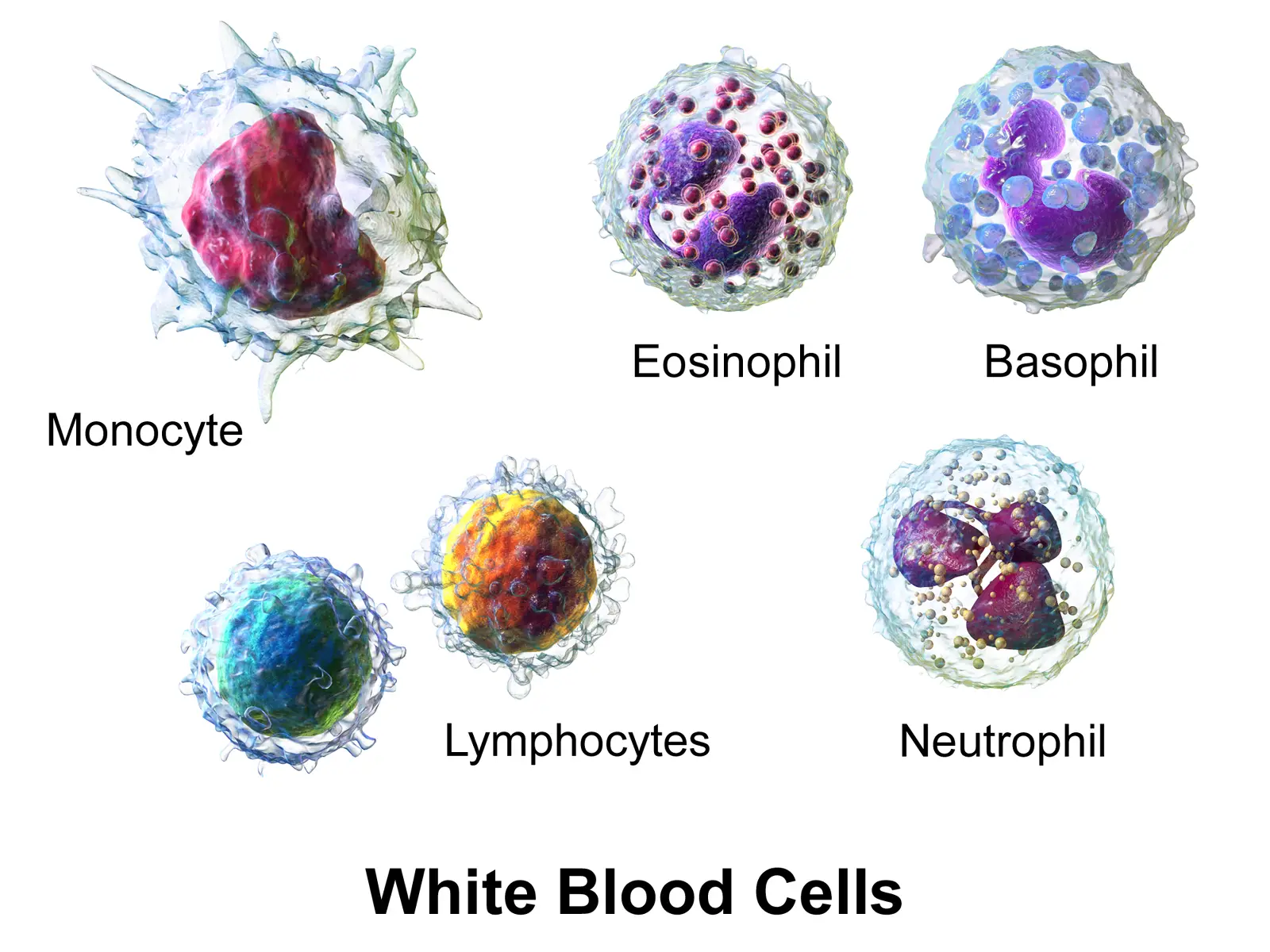
Image: Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014
So why is there still pain after my injury is healed?
Sometimes acute pain from an injury can sensitize peripheral nerves, which can increase nociceptive input into the spinal cord. Consistent input can eventually lead to central sensitization, one of the key factors that helps explain chronic pain even after the injured tissue has healed.
During central sensitization, the neurons in the spinal cord are very sensitive to various types of stimuli, similar to a car alarm that is so sensitive that it blares off when truck roars by. This can trigger a constant false alarm that our nervous system registers as pain. When we’re having chronic low back pain or a throbbing knee pain, nociceptors express and release inflammatory mediators into the spinal cord, such as neurotransmitters, cytokines, and various hormones. These mediators activate microglia, a type of immune cell that plays a big role in central sensitization.
Inflammation sometimes accompanies pain, and it’s not necessarily a bad thing. When our nervous system senses an injury or potential threat that may cause an injury, nociceptors release various mediators that attract immune cells, kind of like an air raid siren that riles up soldiers to their battle stations. They cause tissues to vasodilate and increase tissue permeability, which contributes to tissue swelling and redness (inflammation).
This process also occurs when nociceptors detect bacterial, fungal, or viral infections. However, any disruption of this normal process can lead to various inflammatory diseases in the skin, joints, gastrointestinal and urinary tracts, and respiratory system.
Electrical nerve stimulation produce vasodilation and permeability, which leads to neurogenic inflammation. This is mediated by axon-axon reflexes. Nociceptors release a strong neuropeptide called CGRP and substance P, which also regulate tissue edema. The latter contributes to smooth muscle contractions of lymph vessels by regulating to types of receptors that are associated with the transmission of stress signals, pain, and inflammation— TACR1 and TACR3—which increase permeability and edema formation.
However, it is unknown whether the crosstalk “shapes antigen drainage and adaptive immunity,” Chiu et al. said in their paper. “An interesting question would be to determine whether nociceptor neurons regulate the initiation or priming of T and B cell responses in draining lymph nodes.”
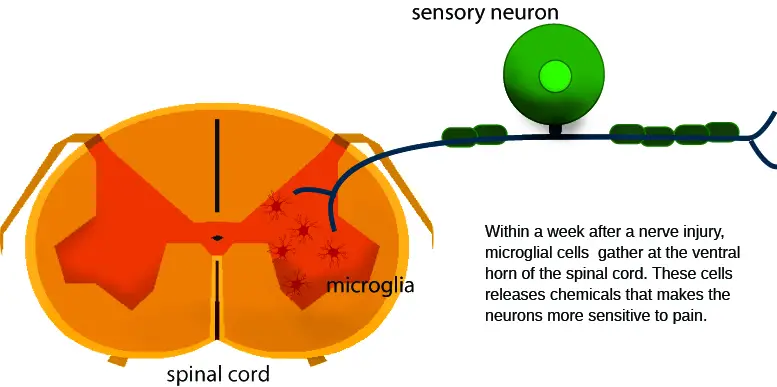
Image: Nick Ng
How microglia and germs affect central sensitization and pain
Microglia are a type of immune cell that reside in the spinal cord. Looking somewhat like the facehugger in the Alien films, they act like a border patrol, keeping a lookout for activities in the neurons that might trigger danger. They produce a neurotrophin — a type of protein — called BDNF (brain-derived neurotrophic factor) that increases sensitivity to nociceptors. Together with T cells, which can infiltrate the spinal cord during chronic pain, they produce various neurotrophins to make us feel even more pain for longer duration.
Not only that, the nervous system crosstalk with the immune cells by producing cytokines and hormones to produce and maintain chronic pain. Microglia can activate nearby glials cells in the spinal cord, such as astrocytes and oligodendrocytes, which are sources of inflammatory mediators. Peripheral nerve injury and sensitization can cause a chain reaction that could lead to chronic pain.
Chiu et al. described one series of reactions after an injury happens:
1. Injury in peripherals releases CX3CL1, a type of cytokine, into the spinal cord.
2. CX3CL1 activates microglia production of TNFα.
3. TNFα activates astrocytes to produce CCL2, cytokine, which activates central neurons, which leads to more pain.
4. Astrocytes make CXCL1, which activates spinal dorsal horn neurons expressing CXCR2 in cancer pain models.
5. Oligodendrocytes produce IL-33, which activates microglia and astrocytes, in mice.
Since bacteria, fungi, and viruses have direct access to invade and infect us, certain parts of our body have a legion of pain-sensitive nociceptors that are constantly detecting pathogens along the epithelial tissues. Because the nervous system can react to danger in nanoseconds — much quicker than the immune system can mobilize its army — it makes sense that these nociceptors are the first line of defense against infections. They can detect substances that are secreted by pathogens, such as lipopolysaccharides (LPS), flagellin, and various bacterial toxins. When these are detected, nociceptors gear immune cells into action while increasing pain sensitivity.
According to Chiu et al., these immune cells can be protective or harmful, depending on the type of infection.
1. Candida albicans
In mice experiments, this infection decreases nociception, which leads to IL-23 (cytokine that mediates in inflammation) production and decreases the host’s ability to fight Candida. However, injecting CGRP (a type of peptide that acts as a vasodilator) in infected mice increases the immune defense.
2. Staphylococcus aureus
Contrary to Candida, nociceptors decreases immune responses, and pain is produced by peptides and bacterial toxins. The decrease in nociception increases neutrophil and monocyte numbers, which heads to the infected site for battle while decreasing lymph node growth. Thus, some types of immune cells are downregulated in order for other immune cells to do their job.
3. Salmonella enterica
By blocking receptor TACR1, this leads to an increase in IgA and Th2 cytokine production, which specialized proteins that provide front-line defense against infections, particularly along mucosal surfaces. This is a mechanism that protects the digestive tract tissues from Salmonella. Sympathetic neurons in the extrinsic layers of the gut are activated downstream from the infection site. This prevents further infections as it travels down the gastrointestinal tract while macrophages gobble up the bacteria.
Based on these evidence, Chiu et al. suggested that any disruption of the normal interaction between the nervous system and immune system could be an underlying cause of such inflammatory diseases of the skin, joints, respiratory system, and digestive tract. “Targeting specific immune cells, cytokines, or lipid mediators may lead to novel approaches to treat chronic pain. Conversely, modulating of nociceptor neuron activity or mediators could lead to new approaches to treat infection and chronic inflammatory diseases,” the authors concluded.
While treating infections is beyond the scope of practice of massage therapists, understanding how the immune, endocrine, and nervous systems modulate pain is important for our decision-making process when providing treatment. Therefore, therapist should not jump to conclusions about why their clients and patients have pain based on the biases of their profession and education.
Further reading
How Your Immune, Endocrine, and Nervous Systems Influence Pain for a Lifetime
A native of San Diego for nearly 40 years, Nick Ng is an editor of Massage & Fitness Magazine, an online publication for manual therapists and the public who want to explore the science behind touch, pain, and exercise, and how to apply that in their hands-on practice or daily lives.
An alumni from San Diego State University with a B.A. in Graphic Communications, Nick also completed his massage therapy training at International Professional School of Bodywork in San Diego in 2014.
When he is not writing or reading, you would likely find him weightlifting at the gym, salsa dancing, or exploring new areas to walk and eat around Southern California.